Steinernema riobrave
H. E. Cabanillas, G. O. Poinar, J. R. Raulston
1994
DESCRIPTION
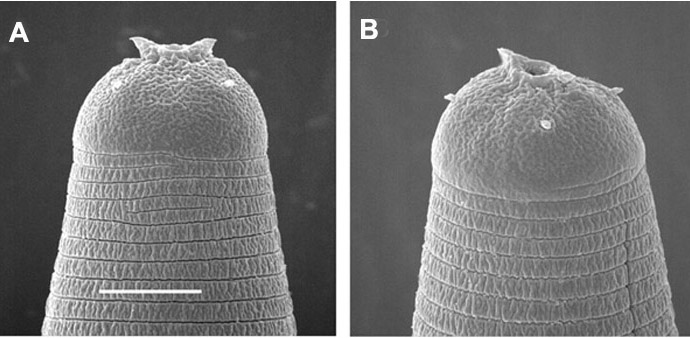
Horn-like structures on labial region of exsheathed infective juvenile
of Steinernema riobrave
Males, first generation:
Generalmorphology (FIG.1),
similar to female. With a single reflexed testis. The spicules are paired,
sickle-shaped, with a distinct dark golden coloration. Spicule head elongated
tapering anteriorly (FIG1.SEM).
Shaft present but short. Lamina moderately curved with two internal ribs
tapering gradually to a bluntly pointed tip. Calornus/larnina angle averaging
100 degrees (range 90-100). Velum present, gubernaculum 0.7 times as long
as the spicules, boat shaped in lateral view and ventrally curved with
a proximal knob or hook. In ventral view, the gubernaculum neck has almost
the same width as posterior corpus. Cuneus Y-shaped or needle-shaped, long,
pointed posteriorly. Twenty five genital papillae (twelve pairs and a single
ventral preanal) were observed under scanning electron microscope. (FIG2.SEM)
Seven pairs are consistently found anterior to cloaca, subventral in position,
pair eight is lateral. A single ventral papilla present consistently just
anterior to cloaca. Occasionally, there is a small additional papilla in
lateral position, near mid-body. Pairs nine, ten, eleven and twelve are
postanal. Pair nine is subventral; pairs ten and eleven are ventral and
subterminal. and pair twelve is subdorsal. The ventral portion of the tail
is usually straight. Anterior flap of cloaca present. Mucron in tail tip
absent.
Measurements (n=10): Length=1,700 um (1,500-1900);
Width=133 um (116-160); EP=103 um (94-111); NR=115 um (106-134); ES=144
um (128-154); testis flexure=226 um (185-257); tail=31 um (29-35); ABW=59
um (50-64); mucron= absent; spicule length=67 um (63-75); spicule width=12.4
um (11.2-13.7); gubernaculum length=51 um (47.5-56.2);gubernaculum width=8.1
um (7.1-8.7); D=0.71 (0.6-0.8)
Abbreviation: um=micrometer; EP=distance from anterior end to
excretory pore; NR=distance from anterior end to nerve ring; ES=esophagus
length; ABW=anal body width; D=distance from anterior end divided by esophagus
length.
Males, second generation:
Similar to but smaller than first generation males.
Females, first generation:
Cuticle smooth, head rounded, continuous with body (FIG.2).
Six distinct lips each with one papilla . Four cephalic papillae. Amphids
distinct, located behind lateral cephalic papillae (FIG2.SEM).
Stoma partially collapsed. Esophageal collar absent, esophagus extending
to near mouth opening. Cheilorhabdions represented by a thickening sclerotized
structure just beneath the lips. Below this, there is another sclerotized
ring that represents the prorhabdions. Meso-, meta and telorhabdions are
vestigial and would occur in the collapsed area of the stoma .
Esophagus muscular with a cylindrical procorporal area followed but
a slightly swollen non-valvated metacorpus, a narrow isthmus, and a basal
bulb with a valve. Nerve ring usually surrounding isthmus. Excretory pore
opening usually anterior to nerve ring, but its location variable. Lateral
fields and phasmids inconspicuous. Gonads amphidelphic, reflexed. Vulva
a transverse slit, usually protruding slightly from the body surface. The
vagina short leading into paired uteri. Eggs deposited initially, but later
hatching inside the females and the juveniles boring their way out. Tail
with rounded projection terminally. Pigmy form occurred in some instances.
Measurements (n=10): Length=6,500 (3,700-8300) um (micrometers),
Width=275 um (200-390), stoma length=5.7 um (4.3-6.3), stoma width=8.0
um (7.1-8.8), EP=96 um (80-118), NR=147 um (131-168), ES=193 um (171-211),
tail length=45 um (41-50), ABW=93 um (63-115), V%=52 (49-56), D=0.49 (0.42-0.62).
Abbreviations: um=micrometer; EP=distance from anterior
end to excretory pore; NR=distance from anterior end to nerve ring; ES=esophagus
length; ABW=anal body width.D=distance from anterior end divided by esophagus
length.
Females, second generation:
Similar but smaller than first generation females. Tailstraight and pointed,
with a prominent postanal swelling.
Infective juveniles:
Body narrower than the corresponding parasitic juvenile. Labial region
with 2 hoen-like structures (FIG3.SEM).
Mouth and anus closed and esophagus degenerate. Tail pointed, usually curved
ventrally when relaxed forming an angle 110 degrees with the body. The
highest number ofincisures in lateral field is eight, the formula of lateral
field is 2, 7, 8, 6, 2 (FIG4.SEM).
The length of the infective stage (n=25) was 622 um (561-701), the greatest
width, 28 um (micrometers) (26-30); distance from the head to the excretory
pore, 56 um (51-64); distance from the head to the nerve ring 87um (84-89);
distance from the head tobase of the esophagus 113 um (I 09-116), length
of the tail, 53.5 um (46-59); width at anus, 16 um (I 5-16.5).
Measurements: (n=20) Body length=622
um (561-701); body width=28 um (26-30); EP=56 um (51-64); NR=87 um (84-89);
ES=114 um (109-116); tail=54 um (46-59); ABW=16 um (15-17);
a=22.5 (20.1-23.5); b=5.4 (4.9-6.0); c=11.6 (10.1-12.4); D%=49 (45-55);
E%=105 (93-111).
TYPE HOST AND LOCALITY
The natural host currently unknown, S. riobrave was recovered from
corn earworms, HelicopervaHeliothis) zea (Boddie) (Lepidoptera:
Noctuidae) that had been placed in soil as trap insects in a corn field
at the United States Department of Agriculture South Farm, in the lower
Rio Grande Valley near Weslaco, Texas, USA. The location where the soil
samples were taken was recorded by GPS (Global Positioning System) receivers.
The Global Positioning System coordinates were latitude 26 degrees 08.155'N,
longitude 97 degrees 57.366'W, and altitude 21.7 m above mean sea level
(Cabanillas et al, 1994).
DISTRIBUTION AND
HOST
Steinernema riobrave has been only recorded from the United States.
This nematode has been found parasitizing prepupae and pupae of Helicoperva
zea and Spodoptera frugiperda (Raulstonet al, 1992). The soil
type where this nematode was isolated was a Hidalgo sandy clay loam (47.9%
sand, 3 5.6% clay, 16.5% silt, 1. 1 % organic mater, pH 8.3, 3 9.95 meq/100
g CEC. Daytime soil temperatures at 5 cm deep during corn growing season
were about 35 degrees C.
BIONOMICS AND HOST
PARASITE RELATIONSHIPS
The life cycle of S. riobrave is comparable to that of other species
of Steinernema. It include the egg, four juvenile stages (separated
by molts) and the adult. The third- stage infective juveniles enter the
hemocoel of the insects deliver their associated bacteria, complete usually
two generations, and then emerge from the insect cadaver as infective juveniles.
Infective juveniles transferred to insect blood drops (G. mellonella
and H. zea) reached the adult stage in 48h and produced eggs in
72 h at 24 degrees C. In Petri dish assays, S. riobrave reached
the preadult and/or adult stages in 48h in fall armyworm larvae (9 day-
old) exposed to infective juveniles at 29.5 degrees C (Cabanillas et al.,
1994). This indicates that the development of this nematode is markedly
influenced by temperature. Experiments in type locality showed that this
nematode can move downward and upward throughout soil profile and survive
well in the dry and hot (35 degrees) environment such as in Texas, USA.
BACTERIAL ASSOCIATE
The mutualistic bacteria associated with S. riobrave was isolated on nutrient
agar and produced a brownish crearn color. The primary form of the bacterium
was characterized by its adsorption of bromothymol blue from NTBA (nutrient
bromothymol blue-triphenyl tetrazolium chloride agar), and adsorption of
neutral red from Mac Conkey agar (red colonies). These characteristics
do not differ from the description of Xenorhabdus nematophilus,
but further studies need to be conducted (Cabanillas et al., 1994).
BIOCONTROL CAPABILITY
The efficacy of S. riobrave to control corn earworm ( Helicoperva
zea) prepupae has been evaluated in greenhouse and field experiments.
It was shown that the efficacy of S. riobrave to control corn earworm prepupae
was influenced by the nematode concentration and placement method under
greenhouse conditions (Cabanillas & Raulston, 1995). These studies
indicated that S. riobrave is effective for suppressing corn earworm
populations, under field conditions of high soil temperature with irrigation
and proper timing (Cabanillas &,Raulston, 1995, 1996). Recently, this
nematode has been commercialized to control different insects in the United
States.
REFERENCE
Cabanillas H. E., G. O. Poinar, and J. R. Raulston
1994. Steinernema riobravis n. sp. (Rhabditida: Steinernematidae)
from Texas. Fundamental and Applied Nematology 17:123-131.
This document was constructed and is maintained by KHUONG
B. NGUYEN
Entomology & Nematology Department
University of Florida

