Steinernema abbasi
Elawad, Ahmad & Reid, 1997
DESCRIPTION
Males (first generation): (Fig. 1).
Body shape and anterior region similar to males of other species of Steinernema
.Excretory pore always anterior to nerve ring, near the end of metacorpus.
Distance from anterior end to excretory pore always more than body width
at excretory pore. Gonads monorchic, testis reflexed. About 60% of males
with normal testis and 40% with reduced or collapsed testis, and distance
from base of oesophagus to anterior end of testis always more than
distance from anterior end of nematode to base of oesophagus. Spicules
paired, golden dark yellow in colour. Spicule head (manubrium) 12-15 micrometers
(um) long, about 20% of spicule length; shaft (calomus) almost absent;
blade (lamina) thick, gradually tapering, about three or four times longer
than head; blade terminus pointed with a depression on ventral side; head/blade
angle ranging between 107-120 degrees; velum present. Each spicule with
two internal ribs; shape of spicule, width of head, and the degree of curvature
variable. Gubernaculum about 70% of the spicule length, boat-shaped, ventrally
curved, slightly swollen in the middle and gradually narrowing distally.
Bursa absent. Twenty-three genital papillae present, with a single large
ventral precloacal one, about 22 um from the cloaca; six pairs of ventrosublateral
precloacal papillae; a pair of ventrosublateral papillae located almost
at the level of the cloaca with four pairs of caudal papillae. Tail short
and conoid, about 60% of the anal body width long with rounded terminus;
terminal mucron absent.
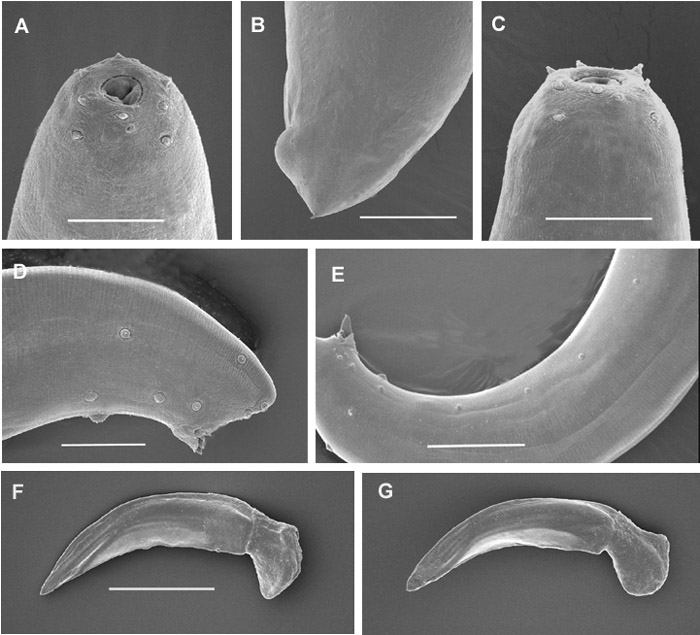
Fig. 1. A:Anterioe region of a female; B: Female tail; C: Anterior region
of a male; D and E: posterior regions of males
showing genital papillae; F and G: spicules.
Measurements:Length=1252
um SD=189 (999-1534); Width=87 um SD=6.7 (82-98); EP=80 um SD=7.8 (82-98);
EPW=45 um SD=3.4 (41-51); NR=103 um SD= 6.5 (99-123); ES=133 um SD=6 (121-144);
testis flexure=274 um SD=33 (134-319); tail=26 um SD=3 (20-31); ABW=43
um SD=5 (37-55); spicule length=65 um SD=5.7 (57-74); spicule width=12
um SD=1.3 (10-14); gubernaculum length=45 um SD=4.3 (33-50);gubernaculum
width=7 SD=0.1 (6-8.5); D%=60 SD=5 (51-68) ;SW=1.56 SD=0.22 (1.07-1.87);
GS=0.7 SD=0.07 (0.58-0.85).
Abbreviations: um=micrometer; SD=standard deviation; EP=distance
from anterior end to excretory pore; EPW=width at excretory pore;NR=distance
from anterior end to nerve ring; ES=esophagus length; ABW=anal body width;
SW=spicule length/ABW; GS=gubernaculum length/spicule length
Males (second generation):
Similar to the first generation except smaller and thinner, with collapsed
testis, spicules and gubernaculum slightly shorter and thinner. Shape of
the spicule and the gubernaculum not different from those of the first
generation males, but slightly variable within the individuals of the same
generation.
Females (first generation):
(Fig. 1). Body shape and morphology structures similar to other species
of Steinernema. Lip region rounded, continuous with the body; preoral
disc present; SEM face view with six labial and four cephalic papillae;
lips amalgamated; amphids small pore-like. Stoma shallow, triangular at
base; oral aperture circular. Excretory pore at the level of metacorpus.
Distance of excretory pore from anterior end always more than the body
width at excretory pore. Gonads amphidelphic, reflexed, often containing
many eggs. Vulva a transverse slit; epiptygma present. Vagina sclerotized,
16 um deep and about 9% of the corresponding body width. Tail short, conoid,
with a pointed tip, about 65% of the anal body width; a ventral postanal
swelling always present.
Females (first generation; giant forms):
Giant females occuring with spiralled body , about three times longer than
the normal female; morphologically similar to normal females.
Females (second generation):
Similar to the first generation females but smaller and tail sharply pointed.
Anal body width about 1.5 of the tail length (?), with ventral post-anal
swelling present.
Infective juveniles: (Fig.
2). Exsheathed infective juvenile with horn-like structures. Body thin,
elongate, sheath (J2 cuticle) present but sometimes lost. Lip region
continuous with body. Lateral fields with eight incisures at mid body.
Excretory pore always weak, near the posterior end of metacorpus. Distance
from anterior end to excretory pore always more than body width at the
same level. Oesophagus with cylindrical procorpus and slightly swollen
metacorpus. Nerve ring just above metacorpus. Tail gradually tapering,
dorsally curved at tip with slight ventral depression.
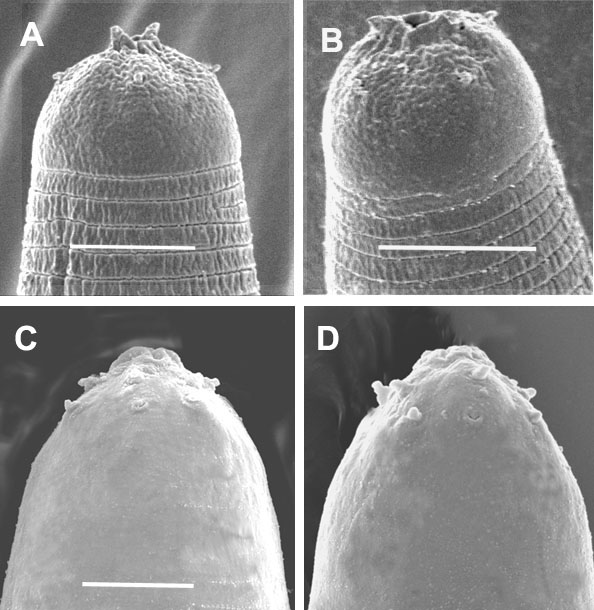
Fig. 2: A and B: Anterior region of exsheathed infective juveniles
showing 2 horn-like structures.
C and D: Anterior region of ensheathed infective juveniles without horn-like
structures.
Measurements:
L=541 um SD=24 (496-579); W=29 um SD=1, (27-30); EP=48 um SD=1.5 (46-51);
NR=68 um SD=2.4 (64-72); ES=89 um SD=1.8 (85-92); tail=56 um SD=3.2 (52-61)
a=18 SD=0.9 (17-20); b=6 SD=0.32 (5.5-6.6); c=9.8 SD=0.8 (8.1-10.8); D%=53
SD=2 (51-58); E=86 SD=5 (79-94).
TYPE HOST AND LOCALITY
Type host: unknown but likely to be a bollworm. Type locality: sandy soil
in alfalfa fields in the Sultanate of Oman (Lat. 16 N and Long 54 E).
TYPE SPECIMENS
Holotype male, two paratype males, two paratype females and two paratype
juveniles deposited at the CABI International Institute of Parasitology
at St Albans, UK.
DIAGNOSIS AND RELATIONSHIPS
S. abbasi can be separated from S. carpocapsae (Weiser),
S.
scapterisci (Nguyen & Smart) and S. riobrave (Cabanillas,
Poinar & Raulston) by morphological, DNA, and hybridization characters.
Male of S. abbasi is similar to that of S. riobrave with
golden dark yellow spicules and the absence of a terminal mucro, but it
has a shorter body length; head/blade angle of the spicule, ranging from
107-120 degrees for S. abbbasi compared to 90-100 for S. riobrave.
Spicule
and gubernaculum length of S. abassi (65 and 45 um) are comparable
to those of S. carpocapsae (66 and 47 um), S kushidai (63
and 44 um), S. riobrave (67 and 51 um) but shorter than those of
S.
scapterisci (83 and 65 um). First generation females of S. abbasi
are similar to those of S. riobrave but the tail shape of
the second generation is wider with a rounded wedge-shaped projection on
the tip compared to the sharp V-shaped tail in S. riobrave.
Diagnostic characters of the third-stage infective juveniles and
adults of S. abbasi do not fit the description of any of the currently
recognized species of the genus Steinernema (Poinar, 1990; Nguyen
& Smart, 1992; Cabanillas et al., 1994). Comparison of the profiles
from S. abbasi and 33 other steinernematid species/isolates held
in a data base at IIP shows it to be a distinct and new species at the
molecular level. S. abbasi is reproductively isolated from S.
carpocapsae, S. scapterisci, and S. riobrave indicated by the negative
results of cross-breeding tests.
BIOLOGY
The life cycle of S. abbasi is comparable to existing species of
Steinernema,
including an egg, four juvenile stages and adults. The third-stage infective
juvenile enters the haemocoel of insects to deliver the associated bacteria
and completes at least two generations before emerging from the cadaver
as infective juveniles. In G. mellonella, adults develop in 48 h
at 25 C and in 36 h at 30 C. S. abbasi appears to be more active
at higher temperatures than S. riobrave as it produces more infective
juveniles in G. mellonella and S. littoralis larvae at 35
C. The LT50 for S. abbasi is slightly superior to that of S.
riobrave against G. mellonella but the temperature profile (thermal
niche breadth for establishment) for S. abbasi is similar to that
of S. riobrave (Elawad et al., 1996). S. abbasi and S.
riobrave
are
clearly nematodes of semi-arid and subtropical regions.
S. riobrave
was recovered from pre-pupae and pupae of H. zea
and S. frugiperda
in Texas, USA (Raulston et al., 1992) and has recently been described (Cabanillas
et al., 1994). S. abbasi is associated with bollworms but it has
not been actually isolated from a known host: it does however reproduce
well in Spodoptera littoralis in the laboratory (unpubl.). It is
likely that S. abbasi could be developed to control pre-prupae and
pupae of bollworms in the Middle East or elsewhere where bollworms are
a major problem, particularly under irrigation.
REFERENCE
Elawad, S, W. Ahmad and A. Reid 1997. Steinernema abbasi
sp. n. (Nematoda: Steinernematidae) from the Sultanate of Oman. Fundamental
and Applied Nematology 20:433-442.
This document was constructed and is maintained by KHUONG
B. NGUYEN
Entomology & Nematology Department
University of Florida


